How to Draw the Lewis Dot Structure for HS- | Hydrosulfide ion
A step-by-step explanation of how to draw the HS- Lewis Dot Structure.
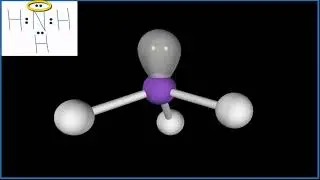
For the HS- structure use the periodic table to find the total number of valence electrons for the HS- molecule. Once we know how many valence electrons there are in HS- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
In the Lewis structure of HS- structure there are a total of 8 valence electrons. HS- is also called Hydrosulfide ion.
--- Lewis Resources ----
• Lewis Structures for Ionic Compounds: • How to Draw Lewis Dot Structures for ...
• Lewis Structures for Covalent Compounds: • How to Draw Lewis Structures: Five Ea...
• Counting Valence Electrons: • Finding the Number of Valence Electro...
• Calculating Formal Charge: • Formal Charges: Calculating Formal Ch...
• Exceptions to the Octet Rule: • Exceptions to the Octet Rule
More chemistry help at https://www.Breslyn.org.
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Watch video How to Draw the Lewis Dot Structure for HS- | Hydrosulfide ion online, duration hours minute second in high quality that is uploaded to the channel Wayne Breslyn (Dr. B.) 21 November 2018. Share the link to the video on social media so that your subscribers and friends will also watch this video. This video clip has been viewed 16,206 times and liked it 78 visitors.
![Сериал [Gacha Life] Две сестры. 1 серия](https://images.reviewsvideo.ru/videos/X1ICrwo2Sy0)