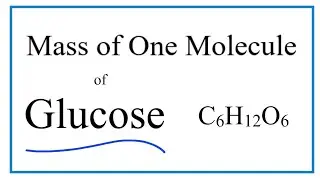
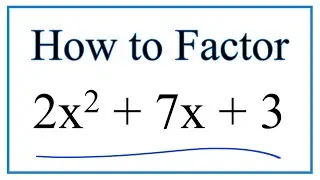How to Find the Mass of One Molecule of Glucose (C6H12O6)
There are two steps to find the mass of a single molecule of Glucose (C6H12O6) in grams. First we find the molar mass for Glucose using the Periodic Table. We then divide this by Avogadro's Number (6.02 x E23).
To find the molar mass for Glucose we add up the atomic masses of the individual atoms in the Methane molecule. Once we have the molar mass for Glucose we can divide by Avogadro's Number to find the mass of a single molecule of Glucose .
You should check your answer to make sure it makes sense. Since molecules are very, very small you should get a very small number as your answer.
Note that this is the mass for a single molecule of Glucose (C6H12O6). In reality electronic balances are not able to measure the mass of one single molecule at a time due to their small size.
For help with molar mass and chemical quantities, the following videos may be helpful:
• More Moles to Grams Practice: • Practice: Converting between Moles an...
• Molar Mass in Three Easy Steps: • How to Calculate Molar Mass (Molecula...
• Understanding the Mole: • Understanding the Mole (the basics)
• Moles - Gram Conversions: • Practice: Converting between Moles an...
• How to Balance Chemical Equations: • How to Balance Chemical Equations in ...
• Mole Ratio: • How to Find the Mole Ratio to Solve ...
• Reaction Stoichiometry: • How to Solve Reaction Stoichiometry P...
My chemistry website: http://www.Breslyn.org
Watch video How to Find the Mass of One Molecule of Glucose (C6H12O6) online, duration hours minute second in high quality that is uploaded to the channel Wayne Breslyn (Dr. B.) 20 July 2022. Share the link to the video on social media so that your subscribers and friends will also watch this video. This video clip has been viewed 41,124 times and liked it 132 visitors.