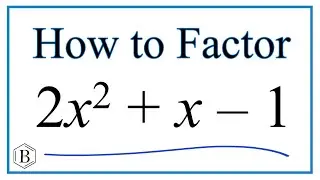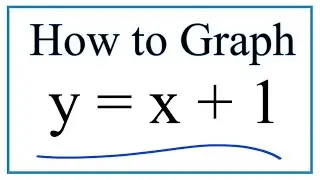How to predict the charge on ions using the Periodic Table.
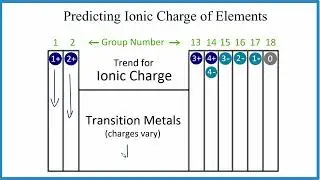
In this video, we'll explore how to predict the charge on ions using the periodic table. By observing a pattern, we can determine the charges of elements when they gain or lose electrons. For example, elements in Group 1 always lose one electron, resulting in a 1+ ionic charge. Group 2 elements have a 2+ charge, while we typically skip the transition metals because their charges depend on what they're bonded to, though they always have a positive charge.
Moving further, elements in Group 13 have a 3+ charge, Group 14 can have a 4+ or 4- charge, and then we go to Group 15 with 3-, Group 16 with 2-, and Group 17 with 1-. Noble gases, in Group 18, are neutral and have a charge of zero.
This trend is a good general guideline, though there are some exceptions. For example, zinc typically forms 2+ ions, and silver usually forms 1+ ions. So, that's how you can predict the charge on ions using the periodic table—just memorize the trend.
Watch video How to predict the charge on ions using the Periodic Table. online, duration hours minute second in high quality that is uploaded to the channel Wayne Breslyn (Dr. B.) 25 August 2024. Share the link to the video on social media so that your subscribers and friends will also watch this video. This video clip has been viewed 1,301 times and liked it 49 visitors.