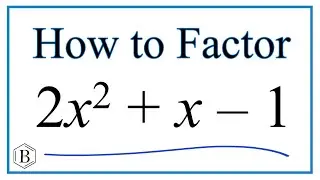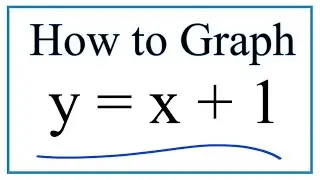Molarity of NaOH (Sodium hydroxide)
To find the molarity (M) for NaOH we use the formula:
Molarity = moles/liters
Often we'll be given the number of moles and liters to find molarity. In this case we divide moles by liters to find the molarity for NaOH (or another solution).
If you are given mL instead of L you'll have to convert milliliters to liters.
If you are given grams instead of moles you need to convert from grams to moles. (See Moles - Gram Conversions: • Practice: Converting between Moles an... )
It is also possible that you will be given the Molarity of a NaOH solution and aske to find the number of moles in a certain amount of liters. Likewise you could be given Molarity and moles and asked to find how many liters would be needed to make the solution.
Watch video Molarity of NaOH (Sodium hydroxide) online, duration hours minute second in high quality that is uploaded to the channel Wayne Breslyn (Dr. B.) 08 June 2022. Share the link to the video on social media so that your subscribers and friends will also watch this video. This video clip has been viewed 65,315 times and liked it 170 visitors.