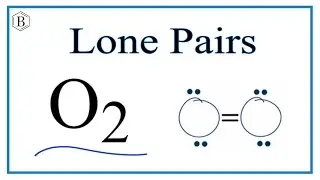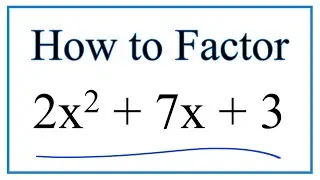Number of Lone Pairs and Bonding Pairs for O2
To determine the number of lone pairs (unbonded pairs) and bonding pairs of electrons for O2 we first need to draw as valid Lewis Structure. Once we have a Lewis Structure for O2 then we can identify the lone and bonding pairs.
O2 Lewis Structure: • How to Draw the Lewis Dot Structure f...
O2 Molecular Geometry: • O2 Molecular Geometry / Shape and Bo...
Bonding pairs of electrons are the electrons between the atoms. These form the chemical bond and are shared between atoms. Often a pair of bonding electrons is represented by a line. Each line represents a pair of bonding electrons.
Lone pairs (unbonded pairs) of electrons are the remaining electrons around the atom. These are not between atoms and are not shared. The are important because they do occupy space and influence the shape of the molecule.
There is one lone pair of electrons on each O atom for O2 (Oxygen gas).
We often need to know the the number of lone pairs of electrons in a molecule like O2 (as well as electrons involved in bonds) to determine the molecular geometry, calculate formal charges, and understand polarity and chemical reactivity.
--Learning Resources--
How to Draw Lewis Structures: • How to Draw Lewis Structures: Five Ea...
Lewis Structures Practice Video Worksheet: • Lewis Dot Structure Practice Problems...
Determining Formal Charge: • Formal Charges: Calculating Formal Ch...
Finding Valence Electrons (molecule): • Finding the Number of Valence Electro...
The Octet Rule: • The Octet Rule: Help, Definition, and...
More help at http://www.breslyn.org
Watch video Number of Lone Pairs and Bonding Pairs for O2 online, duration hours minute second in high quality that is uploaded to the channel Wayne Breslyn (Dr. B.) 04 July 2022. Share the link to the video on social media so that your subscribers and friends will also watch this video. This video clip has been viewed 32,921 times and liked it 202 visitors.