Primary, Secondary, and Tertiary Alcohols: Classification, Examples, & Practice
In this video we'll learn and how to classify alcohols as primary, secondary, or tertiary. We'll also look at examples and practice problems. For more help and practice visit https://www.Breslyn.org !
Because they have different structures 1◦, 1◦, and 1◦ alcohols will have differing properties, such as boiling point.
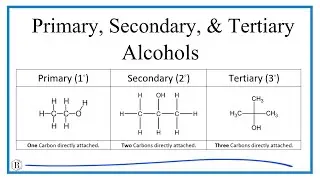
Primary (1◦): One Carbon directly attached to OH group.
Secondary (2◦): Two Carbons directly attached to OH group.
Tertiary (3◦): Three Carbons directly attached to OH group.
Important exception: Methanol, CH3OH, only has one Carbon atom. However, it is counted as a primary alcohol even though there are no Carbon atoms attached to the the -OH Carbon atom.
More explanation about primary, secondary, and tertiary alcohols:
https://chem.libretexts.org/Bookshelv...
Watch video Primary, Secondary, and Tertiary Alcohols: Classification, Examples, & Practice online, duration hours minute second in high quality that is uploaded to the channel Wayne Breslyn (Dr. B.) 19 July 2022. Share the link to the video on social media so that your subscribers and friends will also watch this video. This video clip has been viewed 94,362 times and liked it 1.8 thousand visitors.