How to Write the Name for FeP
In this video we'll write the correct name for FeP. To write the name for FeP we’ll use the Periodic Table and follow some simple rules.
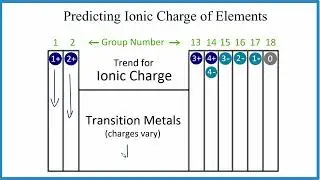
Because FeP has a transition metal we need to indicate the ionic charge using Roman numerals in parenthesis.
--Keys for Naming Compounds with Transition Metals--
1) Write the name of transition metal as it appears on the Periodic Table.
2) Write the name and charge for the non-metal. If you have a polyatomic ion, use the Common Ion Table to find and write the formula and charge.
3) Use the total charge on the non-metal (or polyatomic ion) find the charge on the transition metal.
4) After the name for the metal, write its charge as a Roman Numeral in parentheses. Example: Iron (III) chloride
For ionic compounds like Iron (III) phosphide, you should check to make sure the charges balance to make sure you have the right name.
Naming Resources:
• Simple Ionic Compounds: • Naming Simple Ionic Compounds
• Compounds with Polyatomic ions: • How to Name Ionic Compounds with Poly...
• Compounds with Transition Metals: • How to Name Ionic Compounds with Tran...
• Molecular Compounds: • Naming and Formula Writing for Molecu...
• Naming Acids: • How to Name Acids: Examples and Practice
For a complete tutorial on naming and formula writing for compounds, like Iron (III) phosphide and more, visit:
http://www.breslyn.org/chemistry/naming
Drawing/writing done in InkScape (http://www.InkScape.org). Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Watch video How to Write the Name for FeP online, duration hours minute second in high quality that is uploaded to the channel Wayne Breslyn (Dr. B.) 04 March 2021. Share the link to the video on social media so that your subscribers and friends will also watch this video. This video clip has been viewed 5,197 times and liked it 19 visitors.