How to Draw the Lewis Dot Structure for BO3 3- | Borate Ion
A step-by-step explanation of how to draw the BO3 3- Lewis Dot Structure.
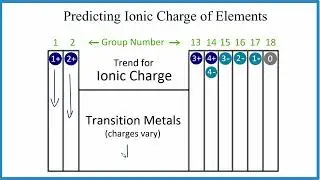
For the BO3 3- structure use the periodic table to find the total number of valence electrons for the BO3 3- molecule. Once we know how many valence electrons there are in BO3 3- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
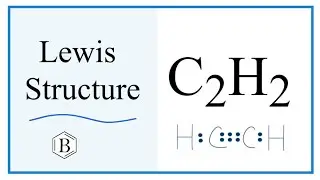
In the Lewis structure of BO3 3- structure there are a total of 18 valence electrons. BO3 3- is also called the Borate ion.
---- Lewis Resources ----
• Lewis Structures for Ionic Compounds: • How to Draw Lewis Dot Structures for ...
• Lewis Structures for Covalent Compounds: • How to Draw Lewis Structures: Five Ea...
• Counting Valence Electrons: • Finding the Number of Valence Electro...
• Calculating Formal Charge: • Formal Charges: Calculating Formal Ch...
• Exceptions to the Octet Rule: • Exceptions to the Octet Rule
More chemistry help at https://www.Breslyn.org.
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet (Bamboo).
Смотрите видео How to Draw the Lewis Dot Structure for BO3 3- | Borate Ion онлайн, длительностью часов минут секунд в хорошем качестве, которое загружено на канал Wayne Breslyn (Dr. B.) 22 Ноябрь 2018. Делитесь ссылкой на видео в социальных сетях, чтобы ваши подписчики и друзья так же посмотрели это видео. Данный видеоклип посмотрели 38,442 раз и оно понравилось 165 посетителям.



![[UNITY] Scary in TERROR game](https://images.reviewsvideo.ru/videos/bUfxg9nBXhg)